Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Monitoring the Emission of Hydrogen from Bacteria in Contaminated Food, Medical Specimens and in Human Blood, infected with Tick-borne Lyme Borreliosis by Gaschromatography and by a Specific Hydrogen Sensor and Examining the Efficacy of Natural Antibiotics
*Corresponding author:Dr. Bruno Kolb, Im Weingärtle 16, 88696 Owingen, Germany
Received:September 19, 2022; Published: September 30, 2022
DOI: 10.34297/AJBSR.2022.17.002327
Abstract
Emission of hydrogen (H2) emerging from microorganisms if cultured in appropriate nutrient medium in closed septum vials are monitored utilizing both headspace-gas chromatography and a specific hydrogen sensor. While obligate aerobic bacteria produce only CO2 and water by aerobic oxidation, facultative anaerobes can emit CO2 and H2 as significant compounds for these classes of bacteria. Obligate anaerobic bacteria emit H2 too but need an oxygen-free atmosphere which can be achieved if the air in the septum vial is replaced by nitrogen. The samples under investigation, either solid or liquid samples and from smears by wads from a cotton bud, are cultured in the headspace vials and the hydrogen emission was monitored after the necessary time of incubation and thus microbe contamination was detected. Antibiotics added to the bacterial culture in the vial are found to be effective if any gas emission is suppressed. If not, they are either ineffective or the bacteria are even resistant. Both pharmaceutical and natural antibiotics were examined, and some were found to be effective or resistant. This method was applied to investigate bacterial contamination of food, household requisite, medical specimens and for the diagnosis and therapy of Lyme- borreliosis caused by tick infection. After infection the responsible borrelia bacteria are detected in ticks and, after transfer to the human body, in blood too. The effect of the antibiotics applied can be examined and the progress of an antibiotic therapy can be controlled until its final success is recognized.
Introduction
Bacteria emit volatile compounds, which are amenable to
analysis by gas chromatography and particularly headspace gas
chromatography (HS-GC) is well suited to analyse such volatiles
emitted from bacterial cultures enclosed in septum vials. Static
headspace gas chromatography was first applied in examining
the growth of bacteria in milk [1] and has found wide applications
in chemistry, physics, food, and medicine whenever volatile
compounds are emitted from complex matrices [2]. The emitted
volatile from bacterial cultures comprises a wide range of organic
compounds (VOCs), but although the composition of the emitted
VOCs contains much information, the pattern produced is in general
not sufficiently informative to identify infectious microbes to the
genus and possibly to the strain level but can be used to detect
bacterial contamination in various samples and to study the efficacy
of natural and chemical antibiotics. Antimicrobial resistance is a
serious issue in medicine that often renders ineffective any effort
to treat patients with bacterial infections [3] and it may become
even worse in the future. Antimicrobial resistance is the ability of
microorganisms to withstand attack by antibiotics. For this reason,
novel antibiotics are needed to treat such multi-resistant microbes.
As an alternative for new pharmaceutical antibiotics there is
much interest in searching for natural compounds with biocide
properties. Such research however requires broad investigations
with many measurements and this need calls urgently for
automation. Automated HS-GC is an established analytical tool and
is well suited for screening applications to process many samples.
This is particularly useful in the search for natural compounds with
antimicrobial properties as an alternative to the increasing number
of multi-resistant chemical antibiotics which have lost their efficacy.
GC is an effective technique to separate a multi component mixture,
a specific sensor may be an alternative if a single compound only
should be detected. The study presented here is focused on the
determination of emitted hydrogen (H2) from bacterial cultures in
closed septum vials and both techniques, static-GC as well as the
sensor approach, are compared in this study. Gas chromatography
has the advantage to automatically process many samples, while
the H2 sensor is a simple and very cheap device and thus sufficient
if few samples only should be investigated. Both techniques have
been used and compared in this work to monitor hydrogen emission
from bacteria for example of the following applications:
a. To recognize bacterial contamination in samples such as
food, household requisites and medical specimens.
b. To examine the efficacy of chemical and natural antibiotics
on bacterial contamination of humans and animals.
c. To study the efficacy of natural antibiotics against
antibiotic resistance.
d. To diagnose bacterial infections on humans on the
example of tick-born Lyme-borreliosis and to control and follow
the efficacy of the appropriate therapy with chemical and natural
antibiotics.
Search of natural alternatives for chemical antibiotics is important. Many natural products with antibiotic properties are known particularly in natural medicine and there is a lot of effort in research, but such products have practically found less application in practical classical medicine. In this work some promising candidates are compared with standards chemical antibiotics and from this limited number both oil of cloves and garlic were found comparable effective with standard antibiotics.
Method and Procedure
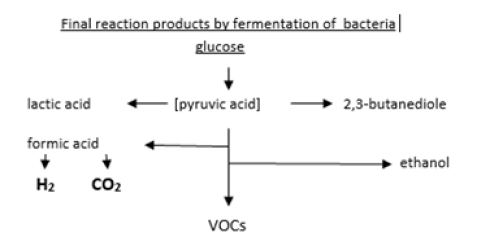
Facultative and obligate anaerobes emit hydrogen (H2) and carbon dioxide (CO2) and volatile organic compounds (VOCs) by fermentation (Figure 1).
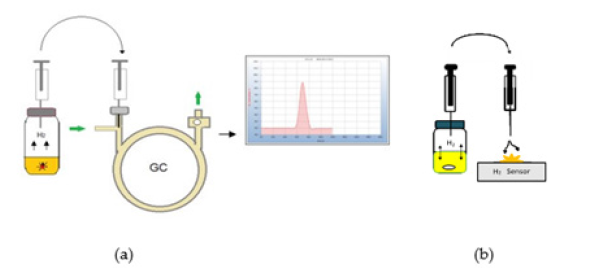
The samples are placed into screw capped headspace vials. The 6ml vials already contain 2.5ml of sterile nutrient medium. After the incubation time a 0.5ml aliquot of the headspace gas is withdrawn by a gas tight syringe by piercing the septum of the vial and is injected into the gas chromatograph and also flushed against the H2-Sensor and a red light indicates the presence of H2 but in addition to this optical response the electrical signal can also be traced by measuring the voltage output (V) and can then be used for quantitative evaluation (Figure 2).
Because this work is focused on the determination of H2 and CO2 just a simple low-cost gas chromatograph for students was found to be sufficient to separate both compounds, although the column could be operated at room temperature only and thus the retention time reproducibility in the following chromatograms therefore may vary slightly with changing room temperature. Since our main interest was the determination of H2, ambient air was used as carrier gas to achieve a highly sensitive response with a positive peak due to the big difference in heat conductivity by the thermal conductivity detector. CO2 on the other hand produces a negative peak at the end of the chromatograms contrary to the positive peak of H2 due to its lower heat conductivity in the flow of ambient air. As an alternative to the GC approach a specific hydrogen sensor was used to proof the H2 emission. In that case an electrical current is created, and a small red lamp lightens while the electrical output (volt) can be used if quantitative evaluation of the H2 emission should be carried out. In the following examples the GC-analysis is compared with the response from the H2-sensor and the result is included in the chromatograms either by the positive (+) or the missing (-) H2 (Figure 3).
Classification of Bacteria
The various classes of bacteria can be identified by their specific composition of emitted volatiles if cultivated in appropriate nutrient media. Obligate aerobic bacteria produce CO2 and water by oxidation of the nutrient medium. Facultative and microaerophile anaerobes can exist both in an air environment as well as under reduced oxygen conditions and can thus emit H2 together with CO2 They start with emitting CO2 by oxidation from the oxygen in the headspace of the vials at the begin of sample incubation but as the oxygen content steadily declines in the atmosphere in the vials the bacteria change from oxidation to the fermentation process, thus producing H2. Obligate anaerobic bacteria produce H2 and many other volatile organic compounds (VOCs) such as free fatty acids, esters, amino acids alcohols and some more, but need an oxygenfree atmosphere.
Interpretation of Chromatograms
Presence of H2 is indicated in the chromatogram by a positive peak and CO2 by a negative peak at the end of the chromatogram. Another negative peak often found at the rear of the hydrogen peak is caused by a change in the gas composition in the headspace vials. Aerobic and facultative anaerobic bacteria consume oxygen to produce CO2 and thus the oxygen concentration of ambient air in the headspace gas of the closed vials is reduced and the nitrogen concentration thus enhanced. This altered gas sample, withdrawn for analysis, has a lower heat conductivity compared with the ambient air used as carrier gas. A negative peak is thus created from the oxygen deficit, which corresponds to the retention time of air on this GC-column. The missing oxygen however is not lost but transferred to CO2, with the corresponding negative CO2 peak at the end of the chromatogram. The chromatograms were obtained using either a GC-column with Silicagel or alternatively with Chromosorb 102. The latter GC-column separates the negative peak of the O2- deficite slightly better from the rear of the H2-peak, but CO2 on the other hand has a longer retention time. However, since these negative peaks may also be caused by CO2 resulting from some oxidation of other compounds in the sample or in the nutrient medium, both negative peaks are not significant for bacteria and are in general not used for this analysis, while the emission of H2 is caused unambiguously by bacteria contrary to the insecurity of the origin of the CO2 emission. Figure 3 presents the various chromatographic patterns with positive and negative peaks and the agreement of a resulting H2 peak with the H2 sensor response.
The corresponding chromatogram no. 1 in Figure 4(a) displays the H2 peak from a contaminated salmon purchased from a supermarket together with the CO2 peak, while a trout caught fresh from a clean river emitted no H2. Both the positive and the missing H2 peak correspond with the H2 sensor response (Figure 4).
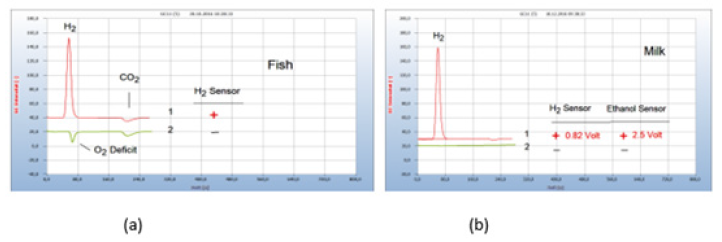
Figure 4:Interpretation of the chromatograms and results. Note*: (a) 1=Salmon purchased from supermarket with hydrogen emission (+); 2=trout caught fresh from a clean river with no hydrogen emission (-). GC-column Silicagel. (b) 1 =raw milk with emission of hydrogen indicated by gas chromatography and by the H2 sensor (+) with an output of 0.82-volt, 2=pasteurized milk with no hydrogen emission (-). Emitted ethanol indicated by a specific ethanol sensor with 2.5-volt output. Milk samples prepared pure without culture medium. GC-column Silicagel.
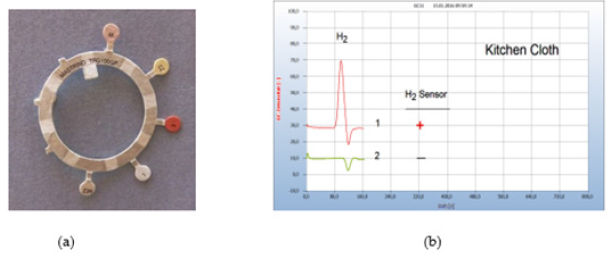
Figure 5:Sensitivity Test. Note*: (a) Antibiotic testing ring TRG 100GP (b) 1=wad from a cotton stick moistened by wet kitchen cloth,2=after addition of 35μg tetracycline taken from an antibiotic testing ring. GC column Chromosorb 102.
HS-GC as well as the sensor approach enable a digital evaluation for further computerized quantitative calculation and documentation contrary to the plate-based techniques of microbiology. Such an example is shown in Figure 4(b). Raw milk apparently contains H2-emitting microbes, often caused by an inflamed udder, and should therefore be consumed pasteurized. The presence of H2 is indicated by a red light at the hydrogen sensor (cf. Figure 3b) and the generated electrical current of 0.82 volt may be used for further computer application. This possibility for further quantitative research however was not yet exploited in this work. The presence of emitted hydrogen is indicated in the chromatograms by the positive sign (+) and a missing hydrogen emission by the black sign (-). The use of specific sensors may be extended to monitor other emitted volatiles from bacteria cultures and such an example is presented also in Figure 4(b) where ethanol was found in raw milk with an electrical output of 2.5volt. Proof of ethanol, however, was not further continued in this work since the presence of ethanol in food or even in blood may not unambiguously be caused by bacteria infection.
Sample Preparation and Incubation Time
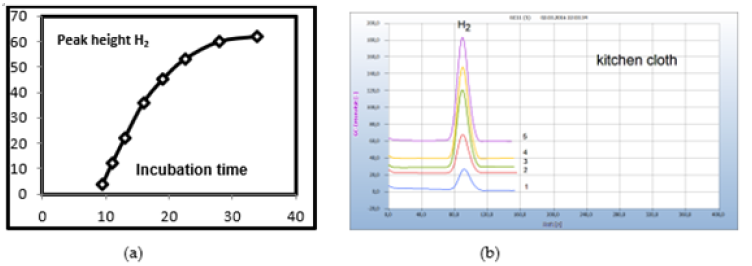
Solid samples are usually sliced and about 50-100mg is placed in the liquid nutrient medium and from liquid samples, 50-100μl. Smears are collected by sterilized cotton buds and the cotton head transferred into the liquid nutrient medium. Several chemical and natural antibiotics were tested and from solid antibiotics few milligrams of the pulverized drug are added to the nutrient medium or one microliter with liquid antibiotics. Since no quantitative investigations have been planned already in this preliminary work the added amount was not quantitatively determined. However, Figure 5 gives an example that even μg amounts of an antibiotic were found already effective. The little plate taken from an antibiotic ring test (Figure 5a) with 35μg tetracycline and added to the nutrient medium containing the cotton bud which was charged with the smear from a wet kitsch cloth worked apparently effective. The wet kitchen cloth used here as an example is known to contain a mixed population of many different bacteria and 360 types of bacteria have already been detected. It is therefore a good source covering a wide range of various microbes to investigate and to compare the general efficiency of pharmaceutical and natural antibiotics (Figure 5).
The samples in the charged vials are incubated at 35°C, and as shown from the kinetic of hydrogen emission in Figure 6(b) a reasonable hydrogen signal is achieved from a sample of a wet kitchen cloth already after an overnight incubation period of about 12h. A constant hydrogen signal required about 30h for this sample. Such a constant hydrogen signal would be required if for quantitative analysis the concentration of the related bacteria should be correlated with hydrogen emission. Due to the difficulty of preparing calibration standards, any quantitative attempts have been omitted so far, particularly because the HS-GC method is preferably used for screening applications. The necessary incubation time, however, can vary with the type of bacteria and may also depend on the original bacterial concentration in the sample, until the necessary amount of H2 is produced by the growing bacteria population. For this reason, we used in general a 2-days incubation time and if H2 or CO2 was not yet found, we repeated the analysis 1 day later to be on the safe side. Some bacteria, e.g., Spirochaetes, need longer incubation due to their slow reproduction, and for such samples, 3 days were sometimes found to be necessary (Figure 6).
Conclusions
Emission of hydrogen from bacteria can be applied to proof obligate anaerobic and facultative anaerobic bacteria but these two classes need different experimental conditions. Obligate anaerobes need an oxygen-free atmosphere in the gas phase of the headspace vials, and this can be achieved if the air in the headspace vial is removed by flushing an already charged vial with nitrogen gas. Such a septum vial filled with nitrogen gas is comparable to a common anaerobic chamber. Facultative anaerobes however are cultured in the headspace vials containing normal ambient air and start with oxidative metabolism but change to fermentation with accompanying release of hydrogen during the declining oxygen content. Both procedures are presented in the following two examples.
Figure 7(a) presents the result of a sample with normal air and on the other hand with nitrogen in the headspace gas. Faeces from carnivores contain obligate anaerobes (Clostridia, Escherichia coli). Small pieces from the faeces from a cat were placed in the culture medium in the headspace vials and the air was removed by flushing with nitrogen to provide the necessary oxygen-free gas phase. The resulting H2-peak no. 1 in the chromatogram emitted from the anaerobic bacteria corresponds with the positive signal (+) from the hydrogen sensor. The presence of oxygen with normal air in the vials (chromatogram no. 2) prevents any generation and emission of H2 by the obligate anaerobes (Figure 7).
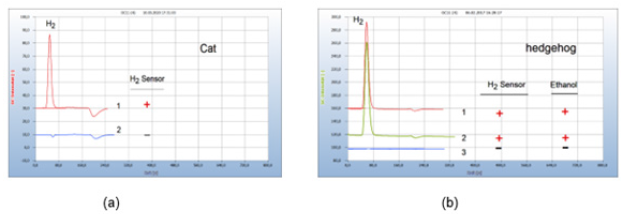
Figure 7:Proof of obligate anaerobes. Note*: H2 - emission of faeces from a cat and from a hedgehog (a) Faeces from a cat; 1=H2 emission from obligate anaerobes under headspace gas nitrogen, 2=under headspace gas air. GC-column silica gel, incubation time 29 hours. (b) Faeces from a hedgehog, 1=H2 emission from obligate anaerobes under headspace gas nitrogen. 2=H2 emission with added amoxicillin, 3=H2 suppression by added clove oil. GC-column silica gel, incubation time 48 hours.
Figure 7(b) presents a similar example of H2 emission from obligate anaerobic bacteria and moreover also the efficacy of a chemical and a natural antibiotic. Again chromatogram no. 1 is from the sample under nitrogen headspace atmosphere. Amoxycillin added to the sample was ineffective to stop emission of H2 (chromatogram no. 2) contrary to added clove oil (chromatogram no. 3). Obligate anaerobes emit not only hydrogen but other volatile compounds (VOCs) too, including ethanol which was identified here with a specific ethanol sensor. Proof of emitted ethanol however was not further exploited in this study because it may not be unambiguous for bacterial infection if found in food or other products.
Results
Bacterial Contamination of Household Requisites
Microbial populations are widely distributed in indoor environments and reside preferably in kitchens and bathrooms. Figure 8(a) presents different hydrogen emissions from a towel, a shaving brush and from a face cloth taken from the bathroom and Figure 8(b) again the result from a kitchen cloth (Figure 8).
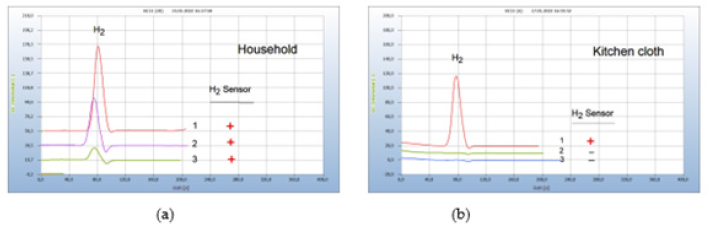
Figure 8:Bacterial contamination of household requisites. Note*: (a) 1= chopped salad packaged in plastic box, 2=bio salad, offered open unpackaged. GC-column Silicagel, incubation time 30 hours. (b) Stability of liver sausage, 1= liver sausage after 10 days in a refrigerator, 2=liver sausage fresh; GC-column Silicagel, incubation time 72 hours.
A smear from such a wet kitchen cloth containing a complex bacterial mixture was prepared and taken to investigate the efficacy of chemical and natural antibiotics. Streptomycin was selected as representative for chemical and chopped garlic for a natural antibiotic. Both antibiotics have eliminated completely hydrogen emission. Apparently, the added garlic was as efficient as the chemical antibiotic streptomycin. Moreover, identical good antibiotic results with the same sample were found with seeds of cress (Lepidium sativum) and with oil of cloves (not shown here).
Bacterial Infection of Food
Food from supermarkets, e.g., fish, turkey, or chicken is often contaminated by microbes. Food poisoning can occur when disease-causing microbes spread to food and if consumed can cause food borne illness. Most food borne diseases are infections caused by a variety of bacteria. Food borne illness usually arises from improper handling, packaging, or food storage. Figure 9(a) presents as such an example the result from two packaged salad samples, one with (chromatogram no. 1) and the other without a hydrogen peak (chromatogram no. 2) the latter declared as biosalad. It is known that chopped salad is found often contaminated by Salmonella bacteria, if stored in the wet environment of closed plastic containers, creating the hydrogen peak, while the bio-salad, offered open and unpacked apparently, was not burdened with microbes (Figure 9).
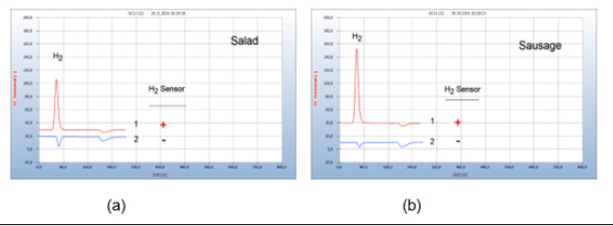
Figure 9:Bacterial infection of food. Note*: (a) 1= chopped salad packaged in plastic box, 2=bio salad, offered open unpackaged. GC-column Silicagel, incubation time 30 hours. (b) Stability of liver sausage, 1= liver sausage after 10 days in a refrigerator, 2=liver sausage fresh; GC-column Silicagel, incubation time 72 hours.
Various bacterial species reside in the kitchen, and in the vegetable compartment of the refrigerators, the major means of food storage within kitchens. Figure 9(b) gives such an example of bacterial infection of liver sausage after storage for 10 days in a refrigerator (chromatogram no. 1), compared with the result of a fresh sausage with no hydrogen emission (chromatogram no. 2).
Food producing animals in poultry or aquatic farms are often contaminated by bacteria. Figure 10 gives an example of the efficacy of standard and natural antibiotics. (a) Trout from an aquatic farm was contaminated apparently with facultative anaerobes as indicted by the H2 peak (chromatogram no. 1). The natural antibiotic oil of cloves (chromatogram no. 4) worked as well as penicillin (chromatogram no. 2) and streptomycin (chromatogram no. 3). Figure 10(b) presents as another such example a comparison between the natural antibiotic seed of cress (Lepidiuim sativum), if added to a salmon from a supermarket with amoxicillin and apparently both drugs worked equally well (Figure 10).
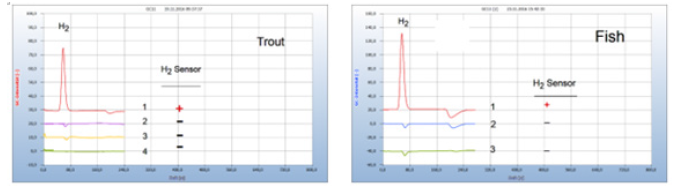
Figure 10:Efficacy of chemical and natural antibiotics. Note*: (a) 1=infected trout, 2=after addition of penicillin, 3=after addition of streptomycin, 4=after addition of oil of cloves; GC-column: Silicagel, incubation time 40 hours. (b) 1 =infected fish, 2=after addition of seed of cress, 3=after addition of amoxicillin; GC-column: Silicagel, incubation time 60 hours.
Test for Antibiotic Resistance
Food producing animals from poultry and fish farming are often contaminated by microbes and there is serious concern on the increasing number of multi-resistant antibiotics. Such examples are shown in Figures 11(a), 11(b). (a) Meat from the leg of turkey (chromatogram no. 1) purchased from a supermarket was contaminated by facultative anaerobes and addition of penicillin (chromatogram no.2) to the culture in a vial was ineffective or the responsible bacteria were already resistant because the H2- emission was still active. Streptomycin and tetracycline, not shown in Figure 11(a), on the other hand were found effective to stop H2 generation (Figure 11).
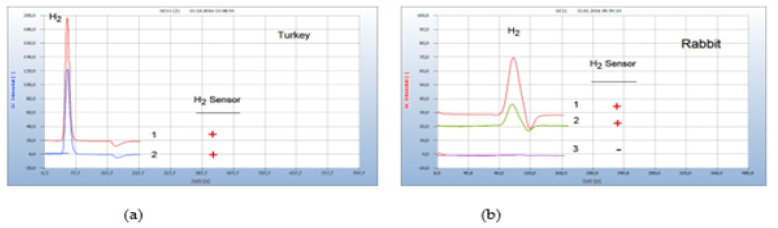
Figure 11:Test for antibiotic resistance. Note*: (a) 1=leg of turkey, 2=after addition of penicillin; GC-column Silicagel, incubation time 50 hours. (b) 1=smear from the inflamed nose of a rabbit, 2=after addition of amoxycillin, 3=after addition of tetracycline; GC-column Chromosorb 102, incubation time 60 hours.
An important application is the search for effective antibiotics in both veterinary and human medicine. Figure 11(b) gives the result of searching for a suitable antibiotic in case of a rabbit with inflamed nose and mouth (chromatogram no. 1). Unfortunately, this animal treated by the veterinary with amoxycillin (chromatogram no. 2) died since apparently amoxycillin was the wrong drug but treated with tetracycline (chromatogram no. 3) the rabbit probably would have survived.
Essential Oils
Essential oils are aromatic and volatile compounds extracted from plants which often possesses antimicrobial properties which have been documented extensively [4]. The following examples present a limited selection of some essential oils with strong activities against pathogenic bacteria, and a comparison with inactive essential oils.
Sliced meat samples of turkey purchased from a supermarket were placed in the vials. The essential oil under investigation was sucked by the sterile wad of a cotton bud and added to the nutrient medium in the vials. Oil of thuja and peppermint were found inactive as was oil of St. John’s wort which is known for certain pharmacological effects. In addition to its anti-depressive effect, St. John’s wort is also assumed to have anti-inflammatory and anti-bacterial qualities. Clove oil and thyme oil on the other hand are found to act as efficient antibiotics contrary to these inactive essential oils, but the real effective ingredients are not known (Figure 12).
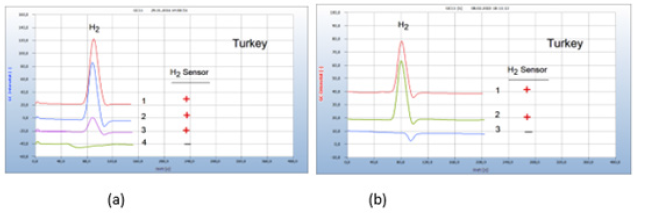
Figure 12:Comparison with inactive and effective essential oils against infected turkey meat. Note*: (a) 1=turkey, 2=added inactive thuja oil, 3=added inactive peppermint oil, 4=added effective clove oil, GC-column Chromosorb 102, incubation time 96 hours. (b) 1=turkey 2=added inactive oil of John’s wort, 3=added effective thyme oil, GC-column Chromosorb 102, incubation time 80 hours.
Application for Lyme- Disease Caused by Tick Infection
Ticks of genus Ixodes are abundant in woodlands and gardens and in some areas up to 25% of ticks contain bacteria Borrelia burgdorferi, and from these about 25% are also supposed to cause infection leading to Lyme disease. In its early stages after a tick bite, pathological skin lesions, often shown as a red ring around the bite, may become visible from a few weeks up to 3 months after the infection and thus often too late for an effective therapy with antibiotics. But in many cases even such lesions are not created, and the infection therefore not recognized. The resulting tick-borne diseases are difficult to recognize since they often arise late after infection and comprise a wide range of various symptoms, such as Lyme arthritis, myalgias, heart abnormalities, chronic fatigue, neuropsychiatric depression and several more. Moreover, the tickborne diseases are not only caused by Borrelia burgdorferi because ticks are often contaminated with a mixture of many other microbes with similar pathogenic characteristics. It is the advantage of the H2 approach to be not specific for Borrelia burgdorferi but comprises all other co-infectious bacteria because Lyme disease is always associated with the complex mixture of co-infections [5]. The result of the H2 approach therefore is given here as Borrelia+ to include all other responsible tick-borne pathogens. The proof of H2 therefore enables a broad range of diagnosis and therapy, not only in vitro but more importantly even in the blood of an infected patient. The emission of H2 from an infected blood sample is thus a direct proof of the bacterial infection contrary to the indirect serological tests (ELISA or WESTERN blot) based on the determination of antibodies only.
However, the absence of Borrelia+ in blood does not necessarily mean that the bacteria are now eliminated, because the bacteria may enter other parts of the human body, such as skin or cartilage, where up to now they cannot be detected due to missing any H2 emission, and not with the standard serological tests either. From these hidden places the bacteria can again become active and may migrate into blood where they can be recognized and combated against. Such regularly repeated attacks are well known with borreliosis and declared as persistent borreliosis. The common serological diagnostics of infected blood are notoriously uncertain [6] and better diagnostics are urgently needed. Moreover, no current test can be used to follow the response to antibiotic therapy.
Monitoring the H2 emission from bacteria cultures in closed vials opens a new approach for diagnosis and therapy of tickborn borreliosis. Borrelia+ are microaerophile and can thrive in an atmosphere with a reduced oxygen concentration compared to air and can change from oxidative metabolism, thus producing carbon dioxide and water to fermentation with hydrogen emission when the concentration of oxygen in the closed vials declines. Figure 13(a) presents the result from 2 ticks, one contaminated by Borrelia+ (chromatogram no.1) and the other (chromatogram no. 2) not contaminated. Two ticks taken from human bodies were introduced into separate vials containing already the nutrient medium and incubated for 2 days. One of the two ticks was contaminated while the other was found free of bacteria as shown in Figure 13(a) (Figure 13).
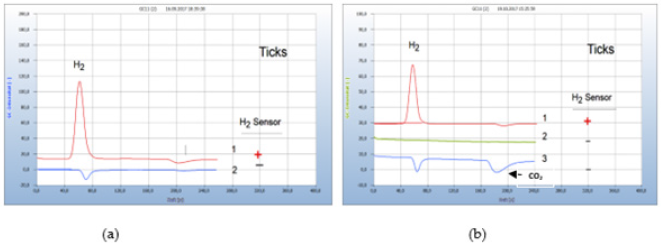
Figure 13:Fakultative anaerobic and obligate aerobic bacteria in ticks. Note*: (a) 1=tick, contaminated with facultative anaerobes (Borrelia+), 2=non-contaminated tick (b) 1=tick, contaminated with facultative anaerobes (Borrelia+), 2=blank from nutrient medium, 3= tick contaminated with obligate aerobes (bartonella?). Incubation time 2 days, GC-column Silicagel.
Case 1: Transfer of Borrelia+ from Tick to Blood
About 25% of contaminated ticks are supposed to cause infection after having attacked a human body since the bacteria are not necessarily transmitted to the human. Only analysis of a blood sample can unambiguously confirm a likely borreliosis infection. However, monitoring the emission of hydrogen does not enable the identification of a single species, such as the responsible Borrelia burgdorferi and the various subspecies but has the advantage that the H2 response comprises the whole complex of co-infections by microbes Ticks may not only be contaminated by Borrelia burgdorferi or some Borrelia+ subspecies because ticks carry a variety of other microbes, e.g. bartonlla, chlamydia, rickettsia and several more, thus causing co-infection and this complicates both diagnosis and therapy. The composition of such mixed infections cannot be recognized by GC-analysis. Only obligate aerobes can unambiguously be recognized if both negative peaks of the oxygen deficit and the corresponding carbon dioxide only are found in the chromatogram but no hydrogen peak. Such an example is shown in Figure 13(b). Two ticks were investigated, and the red chromatogram (no. 1) is from a tick probably contaminated by Borrelia++ while the blue chromatogram (no. 3) apparently is from an aerobic bacterium, probably from bartonella which is the most common bacterium responsible for co-infection. Chromatogram no. 2 is the blank from the pure nutrient medium
Normal healthy blood contains no bacteria Figure 14(a) and the chromatogram (no. 2) is identical with the blank from sterilized wad in the nutrient medium (chromatogram no. 3). Figure 14(b) presents such an example of bacteria transfer into human blood after having been bitten by a contaminated tick. The responsible contaminated tick was collected and proof of Borrelia+ in blood confirmed the actual transfer of Borrelia+ (Figure 14).
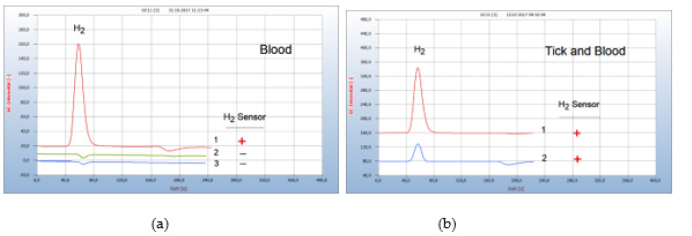
Figure 14:Transfer of Borrelia+ from the tick to blood. Note*: (a) 1=blood with Borrelia+, 2=normal healthy blood without Borrelia+, 3=blank from the wad from a sterilized cotton bud in nutrient media. (b) 1=H2 emission from infected blood, 2=H2 emission from the responsible contaminated tick GC-column Silicagel, incubation time 95 hours.
The GC analysis enables also to investigate the efficacy of various antibiotics. Such investigations, however, have a peculiar problem if various drugs should be tested against a contaminated tick, because for practical reasons it is nearly impossible to cut such a small, infected animal in several equal parts to carry out comparative measurements separately. To tackle this problem a suitable method was created and tested: three ticks were crushed together in about 100μl of the nutrient medium if at least one should be contaminated. From such a homogenous suspension aliquots are transferred to the vials into which the antibiotics to be examined are added.
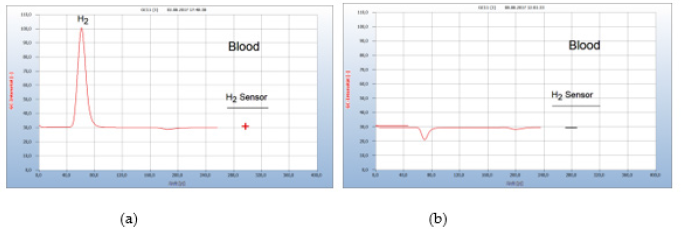
Figure 15:Lyme-disease therapy with doxycclin. Note*: (a) H2 emission from the blood sample 6 weeks after tick bite. (b) blood analysis after 19 doxycycline tablets. GC column Silicagel, incubation 92 hours.
Case 2: The Hydrogen Approach Versus Serological Test
After borreliosis infection was confirmed by analysis of the infected blood a suitable antibiotic therapy should be stated as soon as possible. The most common and most effective antibiotic is doxycycline. However, opinions about the necessary amount of this drug and the length of adequate therapy varies widely between many experts. This is due to the missing control about the effect of such a therapy. Borrelia+ bacteria in blood are in general identified indirectly by serological tests by determining antibodies only, which however once created remain permanently in blood, regardless of whether the therapy was successful. Moreover, these serological tests are notoriously uncertain, and the various therapies have been summarized in a critical review by Marques, et al. [6]. The following example presents such a typical case. Some weeks after having been bitten by a tick, the patient complained about typical tick diseases symptoms such as Lyme arthritis and myalgia. A blood sample was then analyzed by the GC-method and the hydrogen peak (#1) in Figure 15(a) confirmed an infection by Borrelia+ bacteria. The result from a simultaneous serological test however was unclear.
…… antibodies were not evident. In case of only short duration of the clinical symptoms control of the further development it is recommended.
After having thus confirmed the presence of Borrelia+ in blood, a therapy with doxycycline started with 1 tablet doxycycline per day. After 9 days a hydrogen peak indicated that the therapy obviously was still not enough, but after one week later with a total of 19 doxycyclin tablets the hydrogen peak was eliminated and the successful end of the therapy thus indicated Figure 15(b). An additional control analysis a few days later confirmed the successful end of the therapy. Since the emission of hydrogen is a direct and unambiguous proof for the presence of Borrelia+ in blood, the progress of a therapy can be controlled stepwise, and the successful finish thus determined as shown in Figure 15 (Figure 15).
Case 3: Borreliosis-Therapy with the Natural Antibiotic Garlic
Chemical antibiotics such as the preferred doxycycline are mostly administered against borreliosis disease. However, some patients suffer from serious side-effects which are either undesired or even dangerous. The search for safer alternatives therefore is an urgent issue. Some natural products, which are assumed to have antibiotic properties, are recommended for this application and have been examined in this work. These include cardea, cress seed, propolis and some more. From these, two natural products have emerged which were found most effective: oil of cloves and garlic. Oil of cloves, however, can barely be applied, unless the active agent is isolated and identified. Garlic on the other hand is unpleasant at least for many people but is available in the form of various preparations for ingestion, such as pills, capsules, or extracts. In this work capsules with pulverized garlic were examined for their suitability in replacing rough garlic. The antibiotic property of garlic had already been realized by Louis Pasteur. Particularly allicin and related sulfur compounds are known to be able to attack microorganisms. Garlic therefore may be considered as a natural antibiotic that does not damage the gut flora and can support human immune defence since the gut flora is the main source of the human immune system. Garlic is also effective against some antimicrobialresistant bacteria. For all these reasons, garlic was selected in this work to investigate if it can be applied against Lyme-borreliosis. The efficiency of garlic powder was investigated first by an in vitro test. Again 3 ticks were collected, if at least one was contaminated by Borrelia+, and have been crashed together in the vial containing already the nutrient medium to which about 30 mg pulverized garlic powder from a garlic capsule was added. A parallel test was carried out with doxycycline. The result is displayed in Figure 15 and apparently the garlic powder was as effective as doxycycline (Figure 16).
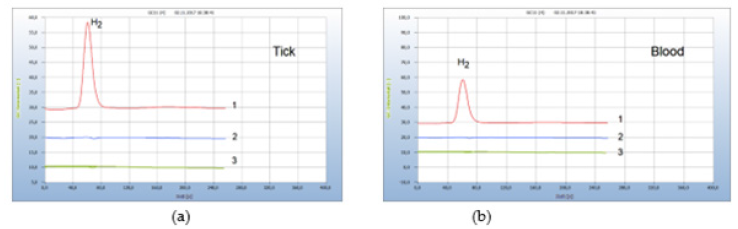
Figure 16:Comparison of the natural antibiotic garlic and pharmaceutical antibiotics. Note*: (a) 1= H2 emission from ticks, 2 =: after addition of garlic powder, 3=after addition of doxycylin. (b) 1= H2 emission from infected blood; 2=after addition of penicillin G; 3=after addition of garlic powder. GC-column Chromosorb 102, incubation 3 days.
The same result with garlic was achieved in the following test with contaminated blood in vitro and Figure 16(b) presents the result. A contaminated blood sample was doped again with garlic powder from a garlic capsule and compared in this case with penicillin G. Again, both the chemical and the natural antibiotic worked equally well.
Case 4: Proof of Borrelia+ in Blood and Efficiency of Garlic Therapy
Following the above pre-tests, a therapy with garlic capsules was started. Examples are given from three patients.
i. Patient #1: Figure 17 again show the results of the therapy with garlic capsules. Already 5 days after therapy regimen with a daily dosage of 900mg (3 capsules à 300mg) the H2-peak was eliminated and thus Borrelia+ in blood either. The therapy was continued with 3 capsules daily and even after 16 days no further Borrelia+ were found in blood (Figure 17).
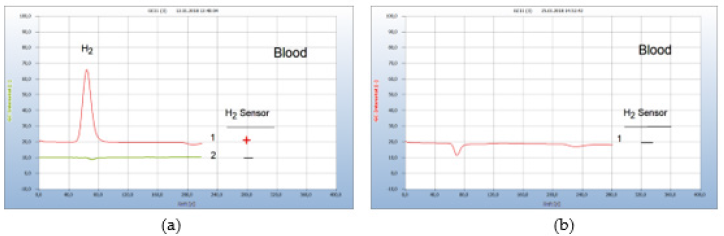
Figure 17:Borreliosis therapy with garlic capsules. Note*: (a) 1=blood before therapy, 2=blood after 5 days therapy. (b) 1=blood after 16 days. CG column Silicagel, incubation 3 days.
iii. Patient #2-Borreliose Therapy with Doxycyclin and with Garlic Capsules: Figure 18 presents the result from a person bitten by a tick and describes the complete course of infection up to a successful therapy. After having been bitten by a tick a red “erythema chronicum migrans” appeared at the upper leg and. The patient suffered from typical health problems although she had not recognized the foregoing responsible tick bite. A blood sample was taken from the vein and a serological test confirmed an infection by Borrelia sensu lato:
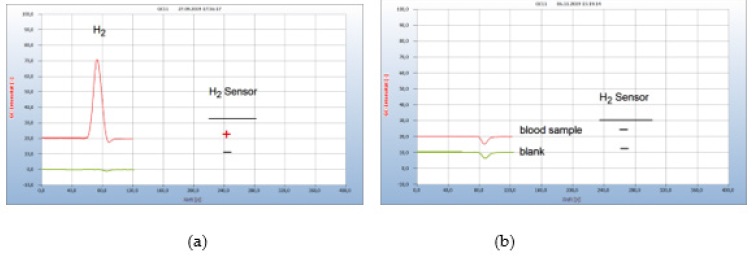
Figure 18:Borreliosis therapy with garlic capsules. Note*: (a) 1= H2 emission from blood after 4 weeks doxycyclin therapy with 200mg/day, 2=blank from cotton bud. (b) 1= blood analysis after 5 weeks garlic therapy with 900mg/day, 2=blankfrom cotton bud. GC column Silicagel
.......the serological result is consistent with an early manifestation of borreliosis
The doctor then applied the usual therapy with doxycyclin, but even after 4 weeks the therapy failed Figure 18(a). The applied daily dose of 200mg was probably not enough, since at least 400 mg is usually recommended by experts. Due to this disappointing result and since the patient did not endure this antibiotic, the therapy was continued with garlic (3 capules/day, á 300mg) and after 5 weeks the H2 signal has disappeared (b) and a repeated analysis some weeks later confirmed the end of this successful therapy (Figure 18).
v. Patient 3: The actual absence of Borrelia+ in blood even after an apparently successful antibiotic therapy does not necessarily guarantee complete bacteria eradication because they may change into inactive round bodies (stationary phase, cysts, L-forms) and enter other parts of the human body such as skin or cartilage, where up to now they cannot be detected neither by the described methods due to missing H2 emission nor with the standard serological tests. From these hidden places the bacteria may change back to the motile helical form and can migrate into blood where they can be recognized and combated again. Such regularly repeated attacks are well known and described as persistent Lyme-disease and are often enhanced if the immune system is weakened by a new infection or by a new disease as shown by the following example.
After a successful borreliose therapy, the patient was free of pain for half a year and a blood test showed no hydrogen peak (not shown here), until he was attacked by a Noro-virus infection. One week after Noro virus infection with the typical virus pains (feaver, diarrhorea) additional pains such as myoalgia typically for Borreliose disease indicated re-growth of Borrelia+ and the (Figure 19(a)) after hydrogen Peak from a blood analysis confirmed the repeated emergence of Borrelia+, apparently due to the weakened immune system. The following 12-day garlic therapy with a daily dosage of 1500mg (5 garlic capsules à 300mg) pulverized garlic powder (peak 2,) was still not enough to eliminate all bacteria. Only after an extended additional 9-day therapy with 900mg/day the bacteria were eradicated in blood. A control analysis after 2 weeks confirmed the successful end of the therapy (Figure 19).
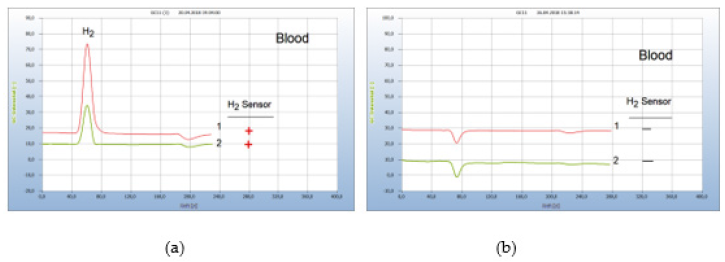
Figure 19:Regrowth of Borrelia+ after Virus infection. Note*: (a) 1=blood analysis after 8 days from beginning of Noro-virus disease, 2=blood analysis after a too short 12 days garlic therapy (b) 1=blood analysis after extended garlic therapy, 2=blank from cotton bud.Conditions: GC column Silicagel. Incubation 96 hours.
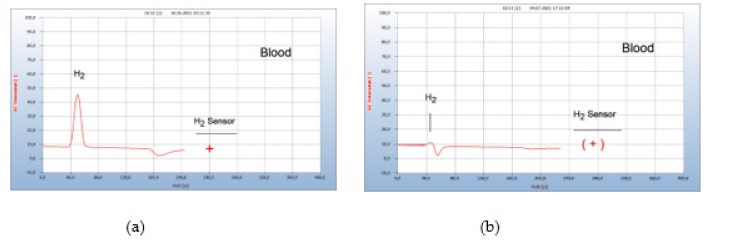
Figure 20:Re-growth of Borrelia+ after vaccination against corona virus infection. Note*: (a) H2 emission from blood sample after vaccination (b) Residual H2 emission from blood sample after 11 days garlic therapy with 900mg/day.
vi. Patient #4-Re-Growth After Vaccination: (Figure 20) The immune system may also become weakened like a virus infection after vaccination due to a stressed immune system causing a re-growth of hidden neuro borreliosis as shown in Figure 20 from a person with a hidden persistent borreliose but with no active Borrelia+ in blood and no pathogenic symptoms for the last half year. After a second vaccination against corona virus the person complained of headache and fatigue and analysis of a blood sample indeed showed a strong reactivation of Borrelia+ (a) in Figure 20. After a garlic therapy of 11 days with 2000mg/day (4 capsules à 500mg) the concentration of Borrelia+ was reduced to about 10% (b) in Figure 20 and after 10 additional days all Borrelia+ were eliminated. The corresponding chromatogram is not shown but was identical with that presented in Figure 19(b).
Discussion
A new method using the emission of hydrogen has been developed to prove the presence of bacteria in various samples, such as food, household requisites and many others and in the blood of patients suffering from borreliosis caused by infected ticks. Food, particularly offered in supermarkets is often contaminated by microbes and requires careful control to prevent the consumers from food-borne diseases. The emitted hydrogen is detected by headspace-gas chromatography and alternatively by using a specific hydrogen sensor. The H2 approach can also be used to study the efficacy of natural and chemical antibiotics and bacterial resistance too. We have investigated a limited number of such natural antibiotics, such as seed of cress, teasel, propolis, tree resin and some essential oils, with varying and not unambiguous success, depending on the sample type, the relevant bacteria, and some other parameters. From these compounds garlic (Allium sativum) and oil of cloves (Eugenia caryophyllus) have emerged as universally applicable natural antibiotics. After garlic was found effective against contaminated food [7] this drug was then applied against Borrelia+ in infected ticks and in the blood of patients suffering from tick-borne Lyme-disease [8,9]. The antiborrelia efficacy of phytochemicals and essential oils was already summarized [10] but has so far not found practical application in medical therapy.
The favourite drug in our study was garlic, applied as capsules, which are more convenient and after all less unpleasant than rough garlic. The antibacterial effect of garlic was already found by Louis Pasteur and described in detail. [3,11]. The Swedish army has applied such garlic capsules successfully as tick-repellents for military personnel [12].
Oil of garlic was found effective in vitro tests even against stationary phase Borrelia burgdorferi together with many other essential oils [13,14]. The proof of contamination by Borrelia bacteria in ticks and their transfer to the blood of a bitten human causing Lyme disease is of particular interest since Lyme disease is a potentially fatal illness. This tick-borne illness comprises serious health effects such as joint malfunctions and neurological or cardiac disorders and many others. If, in its early stages, pathological skin lesions namely erythema migrans appear, such an infection is obvious, but often these lesions are not visible and the infection therefore ignored with fatal consequences. Even if the erythema migrans appears late, sometimes after weeks or months, this may be too late to start an effective therapy because Lyme disease patients can be cured successfully only in the early stage of infection with the common 2-4 weeks antibiotic treatment. If, however, an infection is not recognized due to missing skin lesions Lyme disease progresses and patient continue suffering from chronic symptoms described as post-treatment Lyme disease syndrome and this persistent borreliosis is no longer accessible by the common antibiotics. It is therefore important to start the antibiotic therapy in due time and this requires a reliable diagnosis as soon as possible after a tick bite. The available techniques of diagnosis such as ELISA and Western Blot are not always sufficient reliable to make a definite diagnosis because these serological assays are indirect tests since they measure an antibody response to the infection, not the infection itself and these need 2 to 6 weeks after infection, while the efficacy of antibiotic treatment declines already after 4 weeks [15]. All diagnostic devices available bear major drawbacks in terms of sensitivity and specificity [6] Moreover, these serological tests are not standardized, and antibodies once generated still may remain in blood even if the bacteria are already eradicated. The same argument applies for the PCR technique which identifies residual DNA. A positive PCR test almost always guarantees Lyme infection, but there can be false-negative results if execute in blood either too early or too late. All these indirect diagnostic techniques cannot control the progress of an antibiotic therapy and it is for these reasons why the recommendations on the necessary dosage and the adequate length of therapy vary widely from one week up to several months between many experts due to the uncertainty of the applied therapy. A too long extended antibiotic therapy may damage the microbiome and on the other hand a too early interruption is particularly dangerous. For all these reasons a reliable direct proof of pathogenic bacteria is needed, and such an approach is offered in this study with the H2 technique.
When a host is co-infected, the combined effects of the diseases
act synergistically often causing worse symptoms than a single
infection alone. However, a patient is finally less interested to know
the exact composition of the responsible pathogenic mixture if an
efficient therapy eradicates them all together with the accompanying
pains. Monitoring the H2-emission allows to control the progress of
a suitable therapy until those pathogenic microbes are eradicated
all-together as indicated by the disappearance of the H2 signal.
These results demonstrate that garlic even applied as capsules is
an effective drug against borreliosis, comparable to the common
chemical antibiotics. The necessary length of therapy as given in
the above examples may vary from one week up to several weeks.
The difference might be caused either by different concentrations
of Borrelia+ in blood or on differences in the immune system.
Borrelia+ in blood are not only combatted by antibiotics but on the
other hand also by the individual strength of the immune system,
which may vary widely between individuals. Due to the varying
lengths, each therapy should therefore be controlled regularly until
its successful end is confirmed. It is the advantage of the method
described that it allows complete control of the Borrelia+ infection
just from the beginning with testing already the contaminated tick
until the successful end of a suitable therapy. A systematic diagnosis
and therapy may proceed according to the following procedure:
a. A tick after having been removed from the body is
analyzed immediately as to whether contaminated by Borellia+ and
the result is obtained already between 2 days
b. If the tick has been found contaminated, analysis of the
blood shows whether the infection was transmitted to the blood,
because not in all cases are the bacteria transmitted.
c. If bacteria in the blood are found, a suitable therapy, either
with chemical or natural antibiotics, should start immediately.
d. The progress of the applied therapy is permanently
controlled until its successfully end.
e. Even after the therapy is finished, it is recommended
to repeat this test regularly, particularly in the case of persistent
borreliosis or in the case of re-growth with newly emerging health
complaints.
If this test has confirmed the illness of borreliosis, the physician will in any case begin the therapy with the common and well accepted antibiotic doxycycline. Only in the event of serious sideeffects, which may often strike the patients, an alternative natural antibiotic can help, such as the described garlic therapy. However, other natural antibiotics may also work as well. The garlic capsules used in this work have the advantage that no prescription is required and moreover they are free from unpleasant odours.
Conclusion
The new method to prove the presence of bacteria in various samples by their hydrogen emission using either headspace gas chromatography or alternatively by a specific hydrogen sensor has the unique advantage that the whole procedure is carried out in hermetically closed vials, this already beginning with sample preparation. When the samples are inserted into the culture broth, the headspace vials are capped with PTFE-lined septa and sealed. The septum remains tight, and several gas samples can be analyzed at given time intervals, thus enabling kinetic measurements. After analysis, the closed vials with their contents can be sterilized at the necessary high temperature and then safely discarded. Thus, the personnel in a lab never meet pathogens. Both techniques, HSGC, and the H2-sensor approach, may have different advantages and applications. HS-GC can be carried out with commercially available automated instruments and up to 100 samples thus can be processed unattended. It is therefore ideal for broad range screening purposes. The H2-sensor approach on the other hand is a simple technique and since such a H2-sensor is a very cheap device it is feasible that this technique may be carried out even in the office of a physician or in a pharmacy in case of Lyme disease investigations since no strict safety requirements are afforded.
Both the technique of HS-GC and the H2-sensor approach are perfectly suited for quantitative analysis since both the resulting peak area of an HS-GC analysis and the electrical output (mV) of an H2-sensor lend itself for computer calculation. The real problem for quantitative determination, however, is the preparation of certified calibration standards and this aspect needs further research efforts. Finally, it should be admitted that all examples presented in this article should only be taken just as suggestions to encourage more detailed research in the future to evaluate the proposed method of H2 emission because this research project is finished now and we have no further possibility to continue.
Materials and Instrumentation
Low-cost gas chromatograph GC-AK 11, Aug. Hedinger GmbH & Co KG, Germany. GC-columns: 0.8m x 6mm polyamide tube, packed with Silicagel 60/80mesh and 0.8m x 6mm polyamide tube, packed with Chromosorb 102, 60/80 mesh, room temperature, carrier gas ambient air. Headspace vials: 6ml crimp-capped from PerkinElmer and 10ml screw-thread from Restek, capped with PTFE-laminated butyl rubber septa. Culture medium: CASO-Bouillon tryptic soy broth acc EP+ USP 3080r-20p, Merck Life Science GmbH, Germany, vendor Mibius e.K., Düsseldorf Germany, composition: pancreatic digest of casein, 17g; papaic digest of soya bean meal, 3g; sodium chloride, 5g; dipotassium hydrogen phosphate, 2.5g; glucose monohydrate. Garlic capsules with pulverized garlic, Hirundo Products, FL-9493 Mauren 500mg/capsule with 1mg Allicin, and Kwaiforte300, 300mg/tablet, MCM Klosterfrau Vertriebsgesellschaft mbH, Köln, Germany. Hydrogen sensor: Keyes MQ-8 Hydrogen gas sensor, Modul KS-046, fluxworkshop.
References
- Bassette R, Claydon TJ (1965) Characterization of some bacteria by gas chromatographic analysis of headspace vapors from milk cultures. J Dairy Sci 48: 775.
- Kolb B, Ettre LS (2006) Static Headspace-Gas Chromatography: Theory and Practice. (2nd).
- Patini R, Mangino G, Martellacci L, Quaranta G, Masucci L, et al. (2020) The Effect of Different Antibiotic Regimens on Resistance-A Systematic Review. Antibiotics 9(1): 22
- Hyldgaard, Mygind T, Meyer RL (2012) Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol 3: 12.
- Lantos PM, Wormser GP (2014) Chronic coinfections in patients diagnosed with chronic Lyme disease: a systematic review. Am J Med 127(11): 1105-11110.
- Marques AR (2015) Laboratory diagnosis of Lyme disease: advances and challenges. Infect Dis Clin North Am 29(2): 295-307.
- Kolb BK, Riesterer L, Bier L, Widenhorn AM (2019) Proof of bacteria and the activity of chemical and natural antibiotics by headspace gas chromatography. Journal of Analytical Science and Technology 10: 1-9.
- Kolb B, Schneider EM, Riesterer L, Bier L, Hein T (2019) Metabolic Profiling of Borrelia in Ticks and in Whjole Blood Samples by Headspace Gas Chromatography - A Case Report. Am J Biomed Sci & Res 6(4).
- Kolb B, Riesterer L, Widenhorn AM, Bier L (2020) Monitoring of Hydrogen Emission from Bacteria in Food, Animals and in the Blood of Humans Suffering from Lyme Disease by A Specific Hydrogen Sensor. Antibiotics 9(7): 427.
- Goc A, Rath M (2016) The anti-borrelia efficacy of phytochemicals and micronutrients: an update. Ther adv infect dis 3(3-4): 75-82.
- Harris JC, Cottrell SL, Plummer D, Lloyd D (2001) Antimicrobial properties of Allium sativum (garlic). Appl Microbiol Biotechnol 57(3): 282-286.
- Stjernberg L, Berglund J (2000) Garlic as an insect repellent. JAMA 284: 829-831.
- Feng J, Zhang S, Shi W, Zubcevik N, Miklossy J, et al. (2017) Selective Essential Oils from Spice or Culinary Herbs Have High Activity against Stationary Phase and Biofilm Borrelia burgdorferi. Front Med (Lausanne) 4: 169.
- Feng J, Shi W, Miklossy J, Tauxe GM, McMeniman CJ, et al. (2018) Identification of Essential Oils with Strong Activity against Stationary Phase Borrelia burgdorferi. Antibiotics 7(4): 89.
- Massarotti EM, Luger SW, Rahn DW, Messner RP, Wong JB, et al. (1992) Treatment of early Lyme disease. Am J Med 92(4): 396-403.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.